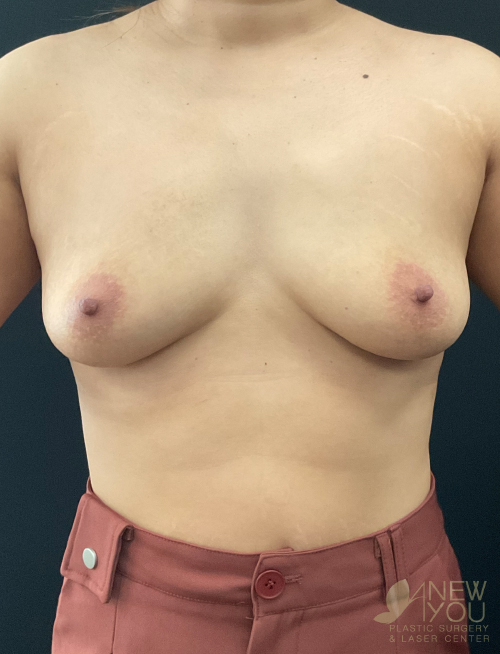
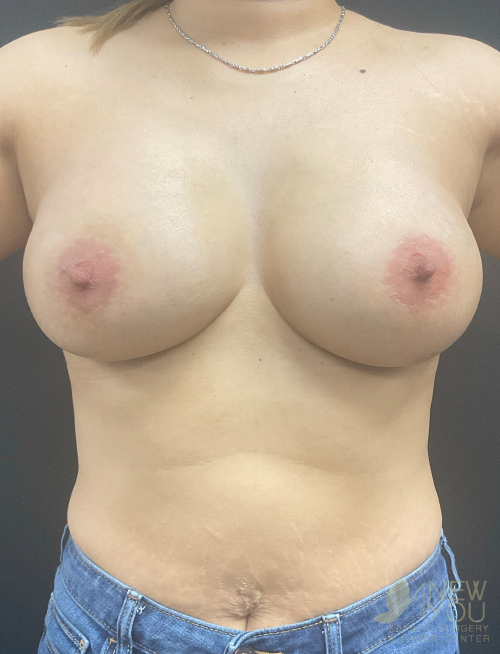
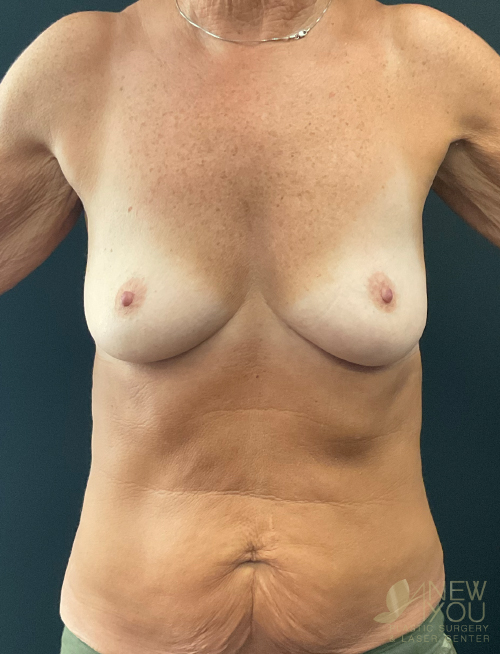
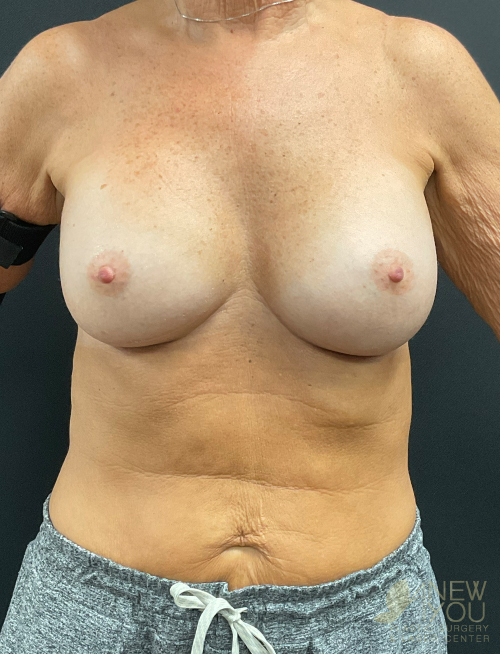
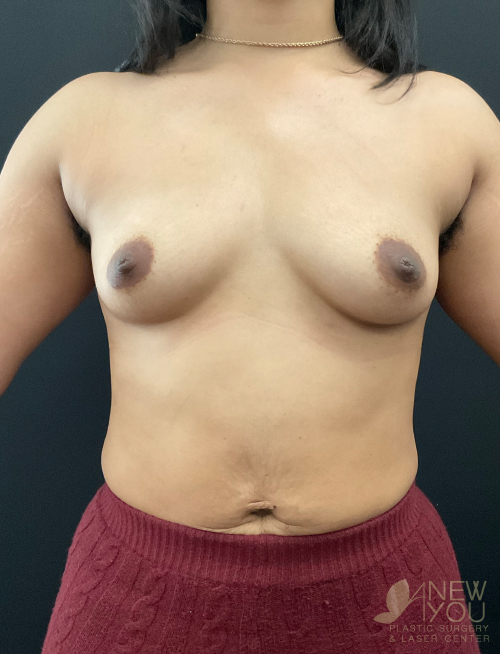
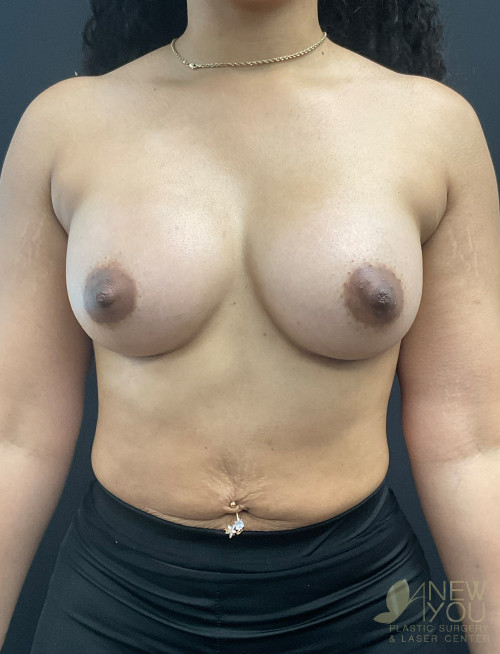
Dr. Samir Shah offers access to a variety of FDA-approved implants from U.S. manufacturers. Saline implants, silicone breast implants, and gummy bear devices are available and come in a variety of sizes to suit your needs.
Saline implants are filled with a sterile saline solution during surgery, allowing for intraoperative size adjustments. If a saline implant ruptures, the body safely absorbs the saline solution, and the rupture is immediately noticeable due to deflation. This provides a safety benefit, as prompt medical attention can be sought. In contrast, silicone breast implants are pre-filled with a cohesive gel and arrive from the manufacturer with a fixed volume, which cannot be adjusted during surgery. Silicone implant ruptures may be more subtle and harder to detect without imaging.
Silicone implants can be considered “gummy” because of the gel. The term “gummy bear” generally refers to highly cohesive, form-stable devices. Firm does not necessarily mean hard, and a degree of “firmness” is often desired to recreate youthful breasts. For women with thin breast skin, a highly cohesive implant is desirable due to a lower chance of rippling. Generally, patients with more breast tissue have greater flexibility in choosing implants. For patients who desire the “public” part of their breast to be more prominent, high-profile round implants create a fuller upper pole. If a natural, subtle upper pole is desired, moderate to moderate plus implants will suffice. Because of the wide range of options available with smooth, round gel implants, these are the most common implants used by Dr. Shah.
In 2017, the 5th-generation smooth round implants were released. Now, Dr. Shah offers 5th generation Allergan’s Inspira line and Mentor’s Memory Gel Xtra, which are highly cohesive (gummy bear), smooth, round implants. In addition to gel styles, implants are available in smooth or textured versions. Currently, all of the shaped implants are textured. If indicated, Dr. Shah may use textured round implants for malposition, augmentation/mastopexy, or when a stable implant position is required. Most importantly, Dr. Shah does NOT use Allergan’s Biocell line of textured implants. As of July 2019, the FDA has requested Allergan to recall all textured implants. Refer to this link to read more information. Currently, Mentor’s textured implants have not been recalled by the FDA.
Just recently, in 2024, the FDA approved a new next-generation implant called Motiva. Motiva breast implants are FDA-approved and recognized for their next-generation design, offering patients new safety features while providing natural-looking aesthetic outcomes. Developed by Establishment Labs, these silicone implants incorporate several key advancements to address the most common concerns related to breast augmentation and implant replacement surgery.
With so many options, the process can be overwhelming. Please call and make an appointment with Dr. Samir Shah to discuss your breast augmentation goals. He will explain all the implant options, and together you will arrive at a decision.